
Abstract
Chronic wounds represent a major public health issue, with an extremely high cost worldwide. In healthy individuals, the wound healing process takes place in different stages: inflammation, cell proliferation (fibroblasts and keratinocytes of the dermis), and finally remodeling of the extracellular matrix (equilibrium between metalloproteinases and their inhibitors). In chronic wounds, the chronic inflammation favors exudate persistence and bacterial film has a special importance in the dynamics of chronic inflammation in wounds that do not heal. Recent advances in biopolymer-based materials for wound healing highlight the performance of specific alginate forms. An ideal wound dressing should be adherent to the wound surface and not to the wound bed, it should also be non-antigenic, biocompatible, semi-permeable, biodegradable, elastic but resistant, and cost-effective. It has to give protection against bacterial, infectious, mechanical, and thermal agents, to modulate the level of wound moisture, and to entrap and deliver drugs or other molecules This paper explores the roles of alginates in advanced wound-dressing forms with a particular emphasis on hydrogels, nanofibers networks, 3D-scaffolds or sponges entrapping fibroblasts, keratinocytes, or drugs to be released on the wound-bed. The latest research reports are presented and supported with in vitro and in vivo studies from the current literature.
1. Introduction
Chronic wounds represent a major public health issue, with an extremely high cost worldwide. In the USA, chronic wounds affect 1% of the total population, in Europe the incidence is 4 million cases per year which implies a rate of about 0.8%, in Australia 0.86%, in China 0.8–1%, and in India 0.6–1% [1,2]. In healthy individuals, the wound healing process takes place in different stages: inflammation, cell proliferation (fibroblasts and keratinocytes of the dermis), and finally remodeling of the extracellular matrix (equilibrium between metalloproteinases and their inhibitors). In the case of chronic wounds, a chronic inflammatory status is noted, so it takes a longer time to reach the cell proliferation and remodeling (healing) phases. Chronic inflammation favors exudate persistence. The bacterial film has a special importance in the dynamics of chronic inflammation in wounds that do not heal. Clinical studies showed that over 60% of chronic wounds presented a biofilm. Current research envisages advanced wound-dressings to address these disadvantages. These wound-dressings should act by removing the biofilm pathogenic bacteria and modulating the inflammation. Many in vitro and in vivo studies centered upon creating new or better biopolymer-based materials for wound healing in recent years. An ideal wound dressing should adhere to the wound surface and not to the wound bed, it should also be non-antigenic, biocompatible, semi-permeable, biodegradable, elastic but resistant, and cost-effective. It has to give protection against bacterial, infectious, mechanical, and thermal agents, to modulate the level of wound moisture, and to entrap and deliver drugs or other molecules [3,4,5]. Alginate, chitosan, collagen, and cellulose are the most used biomaterials for wound-dressing products [3,6,7,8,9,10]. Of these, alginate is by far the most commonly biomaterial among other bioproducts with wound healing properties [3,6,7].
Because of its hydrophilic nature, alginate is capable to take multiple forms [11,12,13,14] (beads, blends, dressings, electrospun scaffolds, flexible fibers, films, foams, gels, hydrogels, injections, microparticles, microspheres, nanoparticles, polyelectrolyte complex, powders, ropes, sheets, sponges) that could be applied on post-traumatic wounds or exuding wounds (ulcers) while decreasing contamination [15,16,17,18], either as a stand-alone biomaterial, or in various combinations.
This review focuses on the roles of alginates in advanced wound-dressing forms with a particular emphasis on hydrogels, nanofibers networks, 3D-scaffolds or sponges entrapping fibroblasts, keratinocytes, or drugs to be released on the wound-bed. The latest research reports are presented and supported with in vitro and in vivo studies from the current literature.
2. Chronic Wounds Mechanisms and Alginates Roles
Wound healing mechanisms involve multiple cellular events, while also being related to the biodynamic of the bacterial film on the wound surface. Inflammation occurs as a result of the inflammatory response of keratinocytes (at the edge of the wound), cytokines, and growth factors during thermal and cellular processes. The cells involved are leukocytes and fibroblasts [19,20,21]. Leukocytes (polymorphonuclear leukocytes-PMN, macrophages, lymphocytes) secrete biomarkers such as IL-1, IL-6, TNF-α, with role for the maintenance of inflammation. Platelets, epithelial cells, endothelial cells, and macrophages secrete growth factors, PDGF (platelets derived growth factors), TGF-β(tumoral growth factor-β), β-FGF(fibroblast growth factor-beta), VEGF-(vascular endothelial growth factor, hypoxia-induced), KGF (keratinocytes growth factors), metalloproteinases-MMPs, and their inhibitors—TIMPs. More than 20 types of matrix metalloproteins have been described to be involved in extracellular matrix (ECM) proliferation [22,23,24].
A fibroblast’s function is to remodel the extracellular matrix and to secrete growth factors. Proliferation is the most critical stage, since the ECM is formed, and the collagen synthesis, reepithelization, and angiogenesis processes begin [25]. Remodeling is represented by the moment when collagen reshapes, the vessels mature and regress from the injured area. Eventually, the reepithelization process takes place [26].
A mechanism implicated in unhealing of wounds seems to point out the fact that fibroblasts are unresponsive to growth factors and cytokines. In patients with chronic wounds, increased levels of IL-1 β, IL-6, TNF-α, and an abnormally high ratio MMPs/TIMPs have been found, as demonstrated by computational models, as well. The liquid in chronic wound with its cytokinic composition seems to inhibit the proliferation of dermal fibroblasts by their arrest in the G0/G1 cell cycle by activating an intracellular molecular pathway mediated by Ras protein [27]: (1) High Mobility Group Box Protein 1 (HMGB1) and the analogues involved in wound repair; (2) cell growth mechanisms regulating given by Ras protein. The study of cell matrix and of the ration between metalloproteinases/their inhibitors seems to have a crucial importance in understanding the chronic wounds physiopathology.
Alginates were proved to exhibit: (1) anti-microbial (Gram-positive—Staphylococcus, Bacillus cereus; Gram-negative—E. coli, Pseudomonas aeruginosa, and Acinetobacter spp. [9,28,29]); (2) antifungal—Candida albicans [9,30,31]; (3) antiviral—Herpeviridae, Rhabdoviridae, Flaviviridae, and Togaviridae, due to sulfated polymeric chain [9,32]; (4) anti-anaphylactic; (5) anti-inflammatory, immuno-modulatory by induction of nitric-oxide (NO), reactive oxygen species (ROS), TNF-α, NF-KB release from macrophages, the MAPK signaling pathway; (6) antioxidant; (7) hemostatic by platelets activation and thrombin clot generation; (8) regenerative/angiogenetic properties [28,29,32,33,34,35,36]. Infection is one of the leading causes for a wound to become chronic [34,37] thus making alginate a good candidate when discussing its possible use as a wound dressing especially in hydrogel forms or more advanced solutions such as electrospun nanofibers networks, 3D-scaffolds and sponges entrapping fibroblasts, keratinocytes, or drugs to be released on the wound-bed.
3. Alginate Physical Properties
Alginates (ALG), are linear water soluble high swelling natural anionic polysaccharides obtained from brown algae cell walls and from some bacteria strains such as Pseudomonas or Azotobacter [6,38,39,40]. They are biopolymers consisting of 1,4-linked β-D-mannuronic acid (M) and 1,4 α-L-guluronic acid (G) monomers [9,30,39,41]. These monomers are grouped in block-like patterns which can be heterogenous (MG) or homogenous (poly-M, poly-G) (Figure 1). When it comes to terminology, alginate usually refer to alginic acid, and its derivatives [6,9,32,42]. To become water soluble for viscous solutions alginic acid should be converted into ALG esters and monovalent salts like sodium alginate or calcium alginate. The viscosity of sodium alginate aqueous solution (1% w/v) for example, is highly dynamic ranging between 20 and 400 mPa·s at 20 °C. By tuning the ALG concentration viscosity and other physicochemical properties are influenced [9,43].
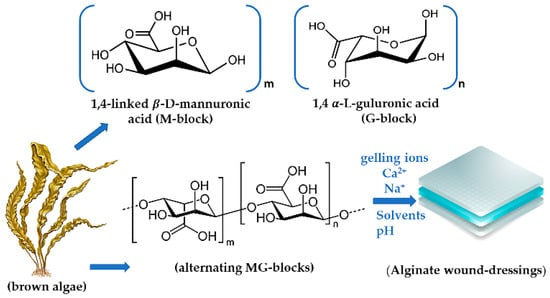
Figure 1. Alginates’ blocks in the polymeric chain.
The parameters to modulate ALG’s solubility are represented by structure, the carboxylic groups states (protonated/deprotonated), ionic strength, concentration, temperature, the amount of the ‘gelling ions’ such as Ca2+ and Na+, the solvents, and pH. At a pKa under 3.28–3.65 the solubility is highly affected and the polymer precipitates [9,32]. ALG’s solubility also changes when long alkyl or aromatic groups are attached to their backbone. Then, the presence of protonated carboxylic groups in ALG’s structure comes with the loss of water or any other solvent solubility. Environmental pH also influences ALG mucoadhesive capacity where the polymers carboxyl groups bind with mucin, and if it is higher, the carboxylic groups become deprotonated [9,15,32,42,44].
The gelling ions trigger a cross-link process of the ALG chains and eventually the gelation process [9,32]. Modulating the G-blocks, M-Blocks, or MG-block concentration in the technological process, different gel patterns can be obtained: stiffer, elastic, or flexible [9,32]. When the alginate is fully crosslinked, the gel will be more rigid, with a higher Young’s modulus and lower elongation which affects its tensile strength. The higher the Ca2+ concentration the better water resistance and swelling behavior is observed, while in thin films more translucent and clear behavior was noticed [9].
With an impressive swelling capacity (20 times their own weight) ALG weakly jellify in the wound environment, providing moisture and stimulating epidermis regeneration [6,9,40]. ALG are acknowledged to have an excellent biocompatibility and it seems that the adverse events were related to the alginate’s (unobserved) impurities that were added unintentionally in the wound-dressings [9,15,42]. The most used alginate types in wound healing studies are the calcium and the sodium alginate, depending on the wound type or the desired dressing form. The physicochemical properties are correlated with the amount of ALG, more ALG will lead to the viscosity and the bead size to increase [43,45,46]. The used concentration of ALG varies from 0.001% w/v to 95% depending on the dressing type [47,48,49].
The ALG wound dressings have the ability of exchanging the ‘gelling ions’ with the wound fluids with a direct application in infected wounds. For example, calcium alginate makes a reliable non-woven wound dressing with the ability to exchange Na+ in exuding or infected wounds. Consequently, this wound dressing type does not adhere to the wound-bed and the removal is painless. The new formed tissue will not be affected by washing away the alginate fibers. Moreover, there is a self-adherence process in the peri-wound area with a good cover of the affected area [9,15].
In the case of sodium alginate salts, only the water solubility is maintained. It dissolves completely in water but not in organic solvents. Nevertheless, sodium alginate has better gel-forming characteristics [9]. To date, at pH of 1.2 spray-dried particles of sodium alginate consisting of hydrophilic matrix controlled-release form, have a longer release time for the entrapped drugs, forming gels in aqueous media. The speed and the drugs’ absorption rate depend of the wound pH and drug type, but also on the solubility of the alginate salt [9,50]. Some authors state that an alginate-based dressing should be changed every week or when the gel loses its viscos properties [51].
4. Alginate-Based Hydrogels for Wound Healing
One of the most promising alginate forms being used in helping wound healing is the hydrogel because it keeps the moisture and absorbs the excessive exudate, it reduces local pain because it has a cooling effect, it does not adhere to the wound bed and it can hold active compounds such as various drugs, signaling molecules, or stem cells. Their disadvantages are their price and their mechanical instability [52,53]. Their structure influences the obtained gel. Repeating M-blocks have a better water retaining ability that transforms into a softer and more elastic gel, whereas repeating G-blocks will give gels a good mechanical resistance, but they will be stiff, and more MG-blocks will lead to a more flexible gel (Figure 2). ALG rich in M-blocks makes soft flexible gels, while ALG in rich G-blocks make firm gels after they absorb wound secretions [11,23,32,42,49,54]. ALG with high G content reveals interesting in situ gel formation properties with superior results after using a gel instead of a solution for ocular drug delivery, being conditioned by pH and temperature [32,55,56]. An oxidized alginate and borax hydrogel dressing obtained directly in situ with a WVTR (water vapor transmission rate) of 2686 ± 124 g/m2/day, was applied on rats, proving that the antiseptic properties of borax helped completely heal the wound within two weeks [57].
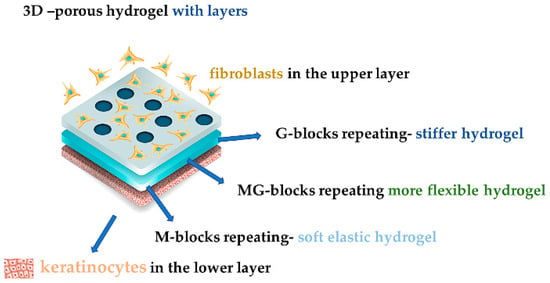
Figure 2. A 3D model of porous hydrogel with fibroblasts and keratinocytes.
If the gelation rate is slow, the gel will be uniform and will have a good mechanical resistance [32,49,54]. To make that happen, one might add phosphate buffer or lower the temperature. Alginate’s gelation rate might be slowed by adding cryoprotectants such as tetrasodium pyrophosphate and di- or trisodium phosphate. ALG also turns into hydrogels after rehydration [32,49,54].
Porous 3D hydrogel calcium alginate (Ca ALG) has great swelling capacity in wounds, providing slow drug release, and it is used to entrap cells for tissue regeneration and engineering, as a physical support for cells or tissue or as a hurdle between two media, because it protects the cells from the host’s immune system until it reaches the targeted area. A great example is represented by the encapsulated fibroblasts into a dual-layered structure made from alginate hydrogel with apical keratinocytes [32,58,59]. Also, a hydrogel film based on poly (N-vinyl caprolactam)-calcium alginate (PVCL/PV-Ca ALG) loaded with thrombin receptor agonist peptide (TRAP) has shown a beneficial effect on wound healing and tissue regeneration [11].
A relatively recent study compared a sodium alginate-acacia gum-based hydrogel loaded with zinc oxide nanoparticles (ZnO-NPs) to only ZnO-NPs by their healing effects and activity against B. cereus and P. aeruginosa. The authors have started from the premise that zinc helps wound healing by having antipathogenic properties, helping reepithelization and reducing the inflammation and bacterial growth in leg ulcers. This study used sheep fibroblasts and concluded that the hydrogel had less cytotoxicity than the use of only zinc oxide nanoparticles, if the concentration is carefully monitored. The hydrogel also demonstrated better results against both aforementioned rod-shaped bacteria. A complete new monolayer was observed if the plate was treated with the hydrogel, whereas the same concentration of only the nanoparticles led to cell death [29]. Also, Neacsu et al. [60] mention a study by Mohandas et al. [61] that concluded that the use of ZnO-NPs in an alginate hydrogel did have antibacterial effects against E. coli and S. aureus, but their used concentration had potentially cytotoxic effects.
When a Na ALG, chitin/chitosan, and fucoidan (60:20:2:4 w/w) hydrogel sheet (ACF-HS) was applied on rats with full thickness wounds in an in vivo cytotoxicity assay study, it provided a moist wound environment, showing easy application and removal, and enhanced cell migration [42,62,63,64,65,66]. The study involved Sprague Dawley rats treated with mitomycin C (wound-healing inhibitor) or Kaltostat® (alginate-based fiber, for the positive control) and ACF hydrogel sheets were applied before being sealed with a plastic sheet [63]. Because the wounds exuded heavily the alginate-chitosan/chitin-fucoidan hydrogel sheets were replaced on day 3. The dressings were removed on day 7 and the established observation period was 18 days. The ACF-HS treated wounds displayed better healing, based on histological examinations. The wound closure and contraction, granulation, capillary formation and re-epithelization started with day 7, whether or not the wound was previously treated with mitomycin C, and the latter process was enhanced after the dressing was removed from the inhibited-healing wound, making ACF-HS a good candidate for wounds with impaired healing [63,64].
Conclusions
The development of alginate-based biomaterials for wound healing has an accelerated pace. The last years gave patients hopes for receiving better treatment for their wounds because the development of a wound dressing that might actually become a true ‘ideal dressing’ seems to be in hands reach. The versatility of alginate-based wound dressings, the promising results after both in vivo and in vitro trials and the cost-effectiveness of obtaining them makes alginate one of the favorites when choosing the material that could act both as a support and as a carrier for the bio-active compounds that have to reach a wound.
References
1. Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major Snoballing Threat to Public Health and Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef][Green Version]
2. Chandran, S.; Seetharaman, A.; Rajalekshmi, G.; Pandimadevi, M. Potential wound healing materials from the natural polymers—A review. Int. J. Pharma. Bio Sci. 2015, 6, 1365–1389. [Google Scholar]
3. Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
4. Erdogan, I.; Bayraktar, O.; Uslu, M.E.; Tuncel, O. Wound Healing Effects of Various Fractions of Olive Leaf Extract (OLE) on Mouse Fibroblasts. Rom. Biotechnol. Lett. 2018, 23, 14217–14228. [Google Scholar] [CrossRef]
5. Miricioiu, M.G.; Niculescu, V.C.; Filote, C.; Raboaca, M.S.; Nechifor, G. Coal fly ash derived silica nanomaterial for mmms-application in CO2/CH4 separation. Membranes 2021, 11, 78. [Google Scholar] [CrossRef]
6. Aramwit, P. Introduction to biomaterials for wound healing. In Wound Healing Biomaterials; Elsevier Ltd.: London, UK, 2016; Volume 2, pp. 3–38. ISBN 9780081006061. [Google Scholar]
7. Liu, J.; Zheng, H.; Dai, X.; Sun, S.; Machens, H.G.; Schilling, A.F. Biomaterials for Promoting Wound Healing in Diabetes. J. Tissue Sci. Eng. 2017, 8, 193–196. [Google Scholar] [CrossRef]
8. Mihai, M. Novel biocompatible chitosan based multilayer films. Rom. Biotechnol. Lett. 2011, 16, 6313–6321. [Google Scholar]
9. Barbu, A.; Neamțu, M.B.; Zăhan, M.; Mireșan, V. Trends in alginate-based films and membranes for wound healing. Rom. Biotechnol. Lett. 2020, 25, 1683–1689. [Google Scholar] [CrossRef]
10. Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Ramos, D.P.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
11. Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
12. Chin, C.Y.; Gan, J.E. Formulation and characterisation of alginate hydrocolloid film dressing loaded with gallic acid for potential chronic wound healing. F1000Research 2021, 10, 451. [Google Scholar] [CrossRef]
13. Snyder, D.; Sullivan, N.; Margolis, B.D.; Schoelles, K. Skin substitutes for treating chronic wounds—Technical brief. In Technology Assessment Program—Technical Brief; Project ID No. WNDT0818. (Prepared by the ECRI Institute-Penn Medicine Evidence-Based Practice Center under Contract No. HHSA 290-2015-00005-I); Agency for Healthcare Research and Quality: Rockville, MD, USA, February 2020. Available online: http://www.ahrq.gov/research/findings/ta/index.html (accessed on 31 May 2021).
14. Koga, A.Y.; Felix, J.C.; Silvestre, R.G.M.; Lipinski, L.C.; Carletto, B.; Kawahara, F.A.; Pereira, A.V. Evaluation of wound healing effect of alginate film containing aloe vera gel and cross-linked with zinc chloride. Acta Cir. Bras. 2020, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
15. Clark, M. Technology update: Rediscovering alginate dressings. Wounds Int. 2012, 3, 3–6. [Google Scholar] [CrossRef]
16. Dumville, J.; O’Meara, S.; Deshpande, S.; Speak, K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev. 2013, 6, CD009110. [Google Scholar] [CrossRef] [PubMed]
17. Radu, C.-D.; Parteni, O.; Sandu, I.G.; Lisa, G.; Munteanu, C.; Lupu, V.V. Specific characterization of a multilayer biomaterial controlled release of tacrolimus. Rev. Chim. 2016, 67, 199–204. [Google Scholar]
18. Samimi, M.; Validov, S. Characteristics of pDNA-loaded chitosan/alginate-dextran sulfate nanoparticles with high transfection efficiency. Rom. Biotechnol. Lett. 2018, 23, 13996–14006. [Google Scholar] [CrossRef]
19. Yussof, S.J.M.; Omar, E.; Pai, D.R.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef]
20. Nagaraja, S.; Wallqvist, A.; Reifman, J.; Mitrophanov, A.Y. Computational Approach To Characterize Causative Factors and Molecular Indicators of Chronic Wound Inflammation. J. Immunol. 2014, 192, 1824–1834. [Google Scholar] [CrossRef][Green Version]
21. Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
22. Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef][Green Version]
23. Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef][Green Version]
24. Matei, A.M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A.; et al. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
25. Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef][Green Version]
26. Orsted, H.L.; Keast, D.; Forest-lalande, L. Basic Principles of Wound Healing An understanding of the basic physiology of wound healing provides. Wound Care Can. 2011, 9, 4–12. [Google Scholar]
27. Ching, C.S.; Phillips, T.J.; Howard, C.E.; Panova, I.P.; Hayes, C.M.; Asandra, A.S.; Park, H.Y. Chronic wound fluid suppresses proliferation of dermal fibroblasts through a Ras-mediated signaling pathway. J. Investig. Dermatol. 2005, 124, 466–474. [Google Scholar] [CrossRef][Green Version]
28. De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967. [Google Scholar] [CrossRef]
29. Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
30. Badea, V.; Paula Balaban, D.P.; Rapeanu, G.; Amariei, C.; Badea, C.F. The antibacterial activity evaluation of Cystoseira barbata biomass and some alginates upon bacteria from oropharyngeal cavity. Rom. Biotechnol. Lett. 2009, 14, 4851–4857. [Google Scholar]
31. Spadari, C.d.C.; Lopes, L.B.; Ishida, K. Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef][Green Version]
32. Szekalska, M.; Pucilowska, A.; Szymanska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef][Green Version]
33. Ueno, M.; Oda, T. Effects of Alginate Oligosaccharides on the Growth of Marine Microalgae. In Marine Algae Extracts: Processes, Products, and Applications; Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH: Weinheim, Germany, 2015; Volumes 1–2, pp. 213–226. ISBN 9783527679577. [Google Scholar]
34. Wiegand, C.; Hipler, U.C. Polymer-based biomaterials as dressings for chronic stagnating wounds. Macromol. Symp. 2010, 294, 1–13. [Google Scholar] [CrossRef





