
Abstract
Wounds have become one of the causes of death worldwide. The metabolic disorder of the wound microenvironment can lead to a series of serious symptoms, especially chronic wounds that bring great pain to patients, and there is currently no effective and widely used wound dressing. Therefore, it is important to develop new multifunctional wound dressings. Hydrogel is an ideal dressing candidate because of its 3D structure, good permeability, excellent biocompatibility, and ability to provide a moist environment for wound repair, which overcomes the shortcomings of traditional dressings. This article first briefly introduces the skin wound healing process, then the preparation methods of hydrogel dressings and the characteristics of hydrogel wound dressings made of natural biomaterials and synthetic materials are introduced. Finally, the development prospects and challenges of hydrogel wound dressings are discussed.
Keywords: wound healing; hydrogels; natural biomaterials; synthetic materials
1. Introduction
The skin is the main defense system of the human body, which can protect the human body from microbial infection and external environmental damage [1,2]. However, due to various internal and external factors, such as physical, chemical, thermal, mechanical, pressure, infection, disease, etc., people often encounter various injuries that cause skin defects. Various wounds of different sizes, depths, and shapes have been formed. They can be divided into acute wounds and chronic wounds according to the injury time [3,4]. Acute wounds refer to wounds that form suddenly and heal quickly. They usually heal by primary healing in cases such as elective surgical incisions, superficial epidermal trauma, and second-degree scalds. Chronic wounds refer to skin tissue injuries caused by various reasons and the healing process takes a long time, such as ulcerative wounds, deep burns, stage III-IV pressure ulcers, diabetic foot ulcers, ulcers caused by radiotherapy and chemotherapy, etc., which are generally more than eight weeks and are easy to repeat [5,6,7]. Wounds have become one of the main causes of death worldwide, causing great inconvenience to human health and economic development [8]. Wound healing generally includes four highly integrated stages which are hemostasis, inflammation, proliferation, and remodeling [3,9,10]. These four stages start in a definite order and last for a period of time, and there may be a partial overlap in time and space between these stages. Creating a clean, moist environment for the wound can accelerate the healing of the wound without inflammation. The ideal wound dressing should have the following characteristics: maintain high humidity; remove excess wound exudate; allow heat insulation; allow gas exchange; fit the wound surface; antibacterial; no fiber shedding/non-toxic, non-adhesive, comfortable, and compliant [11,12,13].
Wound dressings are essential for wound healing as they provide a physical barrier between the wound and the external environment to prevent further injury or infection [3]. Traditional dressings such as gauze may adhere to the newly grown granulation tissue and cause pain when removed. In addition, it has no antibacterial, antioxidant, or other active functions [14]. Therefore, there is a need for biodegradable wound dressings based on bioactive materials that can induce wound healing and promote the deposition of extracellular matrix (ECM). Hydrogels, as hydrophilic gels with 3D network structures, generally have good biodegradability, biocompatibility, adhesion, air permeability, and maintain a moist environment for cell migration, which can effectively promote cell proliferation and facilitate wound healing [15]. These characteristics described above make hydrogels an ideal candidate product for wound dressings [16]. The multifunctional hydrogel wound dressings (such as antioxidative, antimicrobial, and injectable) currently developed can not only provide physical protection and maintain moisture in the microenvironment, but also improve the healing process by affecting the stage of wound repair [17]. For example, antioxidant hydrogels can remove excessive reactive oxygen species in chronic wounds to reduce oxidative stress, thus improving the wound microenvironment, promoting collagen synthesis and re-epithelialization, and reducing the pH value of the wound to accelerate healing and reduce infection [18]. Hydrogels fall into two categories: chemical (permanent) hydrogels that are cross-linked by covalent bonds and physical (reversible) hydrogels that are cross-linked by secondary bonds. Hydrogels can be prepared from natural biomaterials, such as alginate, chitosan, hyaluronic acid, etc.; or synthetic materials, such as polyvinyl alcohol, polyacrylamide, polyethylene glycol, etc. [19,20]. Therefore, this article reviews the physiological process of wound healing, and it focuses on the preparation methods of hydrogel dressings and the characteristics of hydrogel wound dressings made of natural biomaterials and synthetic materials. Finally, the development prospects and challenges of hydrogels are discussed.
2. The Physiological Process of Wound Healing
The skin is the main external defense system, which protects the body from microbial invasion and the influence of the external environment. Therefore, skin damage can pose a serious threat to human health [12]. The skin is composed of three tissue layers: the epidermis, dermis, and hypodermis [21]. The most important function of the epidermis is to form the external barrier of the body, as well as having absorption and immune functions. The dermis contains fibroblasts, mast cells, lymphocytes, and so on. Fibroblasts can produce collagen fibers, elastic fibers, reticular fibers, and matrices. At the same time, it is the main tissue repair cell after the deep damage of skin tissue. The hypodermis has loose tissue and rich blood vessels, which have the functions of connecting, buffering mechanical pressure, storing energy, and maintaining heat preservation. Knowing the composition of normal skin is helpful to our understanding of wound healing. Globally, chronic wounds impose a significant burden on patients and healthcare systems. Chronic wounds are susceptible to bacterial invasion, which can form biofilms at the wound site and inhibit the proliferation of endothelial and epidermal cells. Serious cases can be life-threatening [22,23].
The wound includes the surface, the base, the cavity, and the wound margin, which is collectively called the wound bed [24]. After the body is injured, various coagulation factors play a role to achieve the purpose of hemostasis; cells near the wound secrete growth factors and cytokines to attract fibroblasts, immune cells, and endothelial cells to activate the healing cascade [25]. In the stage of inflammation, the blood vessels relax, thereby increasing the permeability of the blood vessels, and various inflammatory cells gather near the wound bed. Many inflammatory factors, such as IL-1, TNF-α, TGF-β, etc., activate lymphocytes, monocytes, and macrophages to clear pathogens and debris, and prepare for the formation of granulation tissue. A large number of growth factors and cytokines are released to promote cell proliferation and migration [15]. During the proliferation phase, many growth factors, such as FGF, VEGF, EGF, play an important role in promoting cell proliferation. The endothelial cells grow rapidly and induce the formation of blood vessels in the granulation tissue, the damaged tissue is gradually replaced by epithelial cells and fibroblasts, and the wound surface is gradually filled with granulation tissue. Maturation is the final stage in which connective tissue is formed and the new epithelium is strengthened [26,27]. Figure 1 shows the physiological process of wound healing.
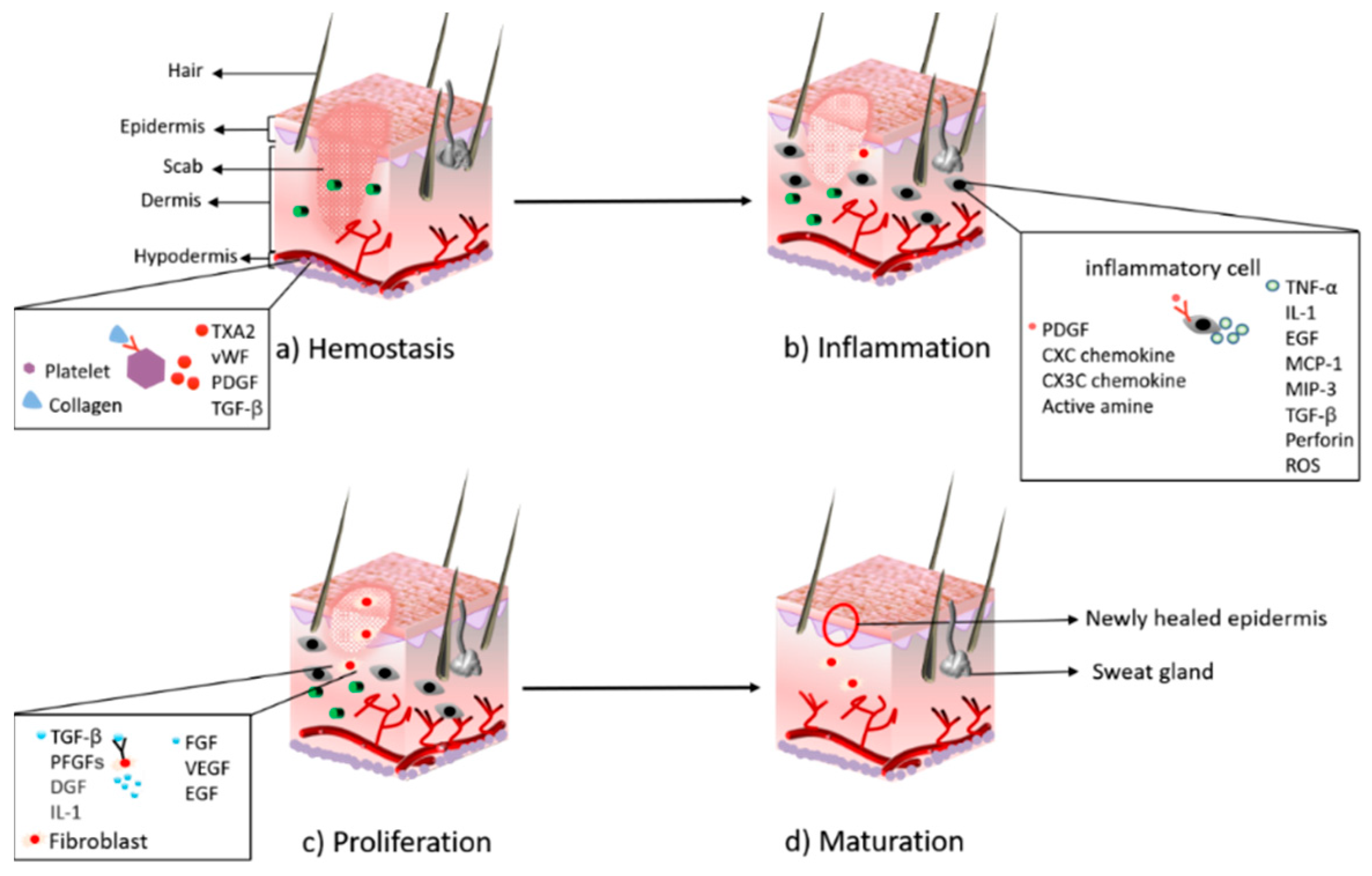
Figure 1. The major stages during the process of wound healing: (a) hemostasis; (b) inflammation; (c) proliferation; (d) maturation.
3. Preparation Method of Hydrogels with a Promising Treatment for Wound Healing
Different hydrogel wound dressings are selected according to different wound conditions. An ideal hydrogel wound dressing should have the following characteristics: (i) it should have biocompatibility and blood compatibility, and hydrogels should be able to stop bleeding immediately and stimulate hemostasis-related factors to act to promote wound healing [28]; (ii) hydrogels should have sufficient adhesion and excellent mechanical properties, even under humid and dynamic conditions they can adhere to and completely seal wounds to prevent bacterial infections [29]; (iii) good moisture retention, providing moisture to the wound site, maintaining a moist environment for cell migration, and promoting cell proliferation [30]; (iv) it can be completely degraded after a period of time, and no by-products are produced [31]. At present, the preparation of hydrogels is mainly divided into physical cross-linking and chemical cross-linking. The effect of cross-linking determines the physical and chemical properties and functions of the hydrogel dressing. Figure 2 shows some preparation methods. The wound microenvironment is affected by many factors, and hydrogel characteristics play a major role in maintaining a favorable microenvironment. According to the required conditions, different preparation methods are selected [32].
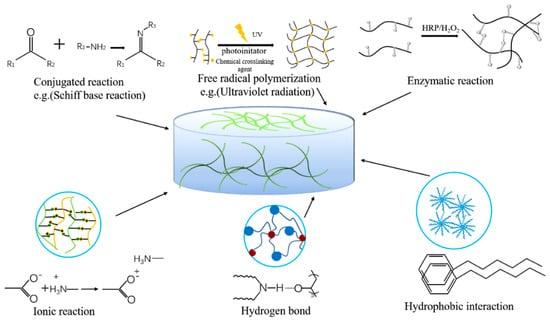
Figure 2. Cross-linking methods of hydrogels.
3.1. Physical Cross-Linking
Most hydrogel dressings with toughness and a high self-healing ability are generally formed by non-covalent bond polymerization. The 3D network structure of the hydrogel dressing formed by physical cross-linking is mainly formed by the interaction between molecules.
3.1.1. Ionic Interaction
Based on the dynamic interaction between oppositely charged groups or metal-ligand interaction is an effective way to carry out ionic interactions [33]. The hydrogels formed by ionic interaction have good ionic conductivity, fatigue resistance, environmental response, and self-healing ability. However, the poor mechanical properties and complex preparation process of hydrogels formed by ionic interactions are still the main problems that prevent their further application [31]. At present, more and more researchers are focusing on designing new hydrogels to solve these problems. For example, negatively charged monomer acrylic acid (AAC) and positively charged 2-hydroxypropyl trimethylammonium chloride chitosan (HACC) through ion interaction to form a high-density dynamic ionic bond of the compact structure of PAAC/HACC hydrogel. The structure endows the hydrogels with good mechanical properties, ionic conductivity, and good self-healing properties, the ionic conductivity is adequate to transfer bioelectrical signals and electrical stimulation on the cell proliferation and differentiation in the human body [34]. Liu et al. prepared CNF/G/Ag0.5 interpenetrating polymer network hydrogels (IPN) through dynamic ionic bond cross-linking. The hydrogels adhered to the surface of the wound and led to platelet aggregation. Gelatin can promote the erythropoiesis, and increase the number of platelets and white blood cells to blockade bleeding and the moderate cross-linked hydrogels could increase water absorption efficiency and decrease the water vapor diffusion, and as a consequence, decreased the WVTR to keep an appropriate balance of fluids on the wound bed [35].
3.1.2. Hydrogen Bond
The use of hydrogen bonds is often indispensable, and the self-repair and self-recovery capabilities of hydrogels can be greatly improved through hydrogen bonds [36]. However, because hydrogen bonds are often unstable in aqueous environments, the resulting hydrogels often have low utilization rates. Currently, researchers have improved the effect of hydrogen bonding by preparing DN hydrogels or IPN hydrogels [37]. Bi et al. constructed physically cross-linked chitosan-polyvinyl alcohol DN hydrogels based on multiple hydrogen bond interactions. Since the hydrogen bond interaction is a dynamic interaction, the hydrogels can be spontaneously rebuilt after being destroyed. In addition, the hydrogels prepared by physical cross-linking have good cell compatibility and biodegradability [38]. Zhao et al. used hydrogen bonds to promote the self-assembly of SA in the PAM porous matrix. PAM-SA semi-interpenetrating polymer network hydrogels not only have good mechanical properties but also have good self-healing efficiency at room temperature based on hydrogen bond interaction. This property can prolong the hydrogels’ lifespan during their applications, especially in a severe environment [39].
3.1.3. Crystallization
The freeze–thaw method is one of the commonly used methods of physical cross-linking. In the freezing part of the cycle, the formed ice crystals arrange the polymer chains around themselves. Then, during the thawing process of the cycle, the ice crystals melt to form a microporous structure [40]. The time, temperature, number of cycles, and the content of polymer components can be controlled during the freeze-thaw process for different pore sizes, mechanical strengths, morphology, or other characteristics [41,42]. The soft, flexible, and variable pore hydrogels prepared by the freeze-thaw method can simulate ECM, and the stem cells mounted on it can sense and respond to the dynamic changes of ECM stiffness, and respond and move in a directional manner, which is crucial for recruiting cells for wound healing [43]. By changing freeze-thaw conditions, PVA/HA hydrogels with a wide range of stiffness spectrums can be useful dressings for basic research related to stem cell differentiation, reprogramming, cell migration, and tissue regeneration [44]. The study of Bor et al. showed that increasing the number of F/T cycles will cause the PVA hydrogels to become hard, thus facilitating the release of drugs, which in turn accelerates wound healing [45].
3.1.4. Hydrophobic Interaction
Hydrophobic interaction is a strong and stable physical interaction, which is a method of cross-linking hydrogels in water-soluble polymers with hydrophobic end groups, hydrophobic side chains, or hydrophobic monomers [46]. The mechanical properties of hydrogels can be improved by incorporating hydrophobic units into hydrogels by chemical or physical methods [47]. Su et al. introduced hydrophobic alkyl groups into PAAm and PAAc hydrogels to enhance the adhesion of hydrogels so that the hydrogels will not easily fall off from the wound due to sweating and rubbing [48]. The use of hydrophobically modified gelatin (HMG) as a hydrogel material has great potential as a carrier for charged hydrophilic therapeutic drugs (bFGF) and hydrophobic drugs (fluorescein sodium). As hydrophobic adsorption is reversible, drugs should be gradually released from HMG hydrogels in vivo to satisfy the adsorption equilibrium because released drugs diffuse out into the body fluid, thereby promoting angiogenesis [49]. The mechanical properties of hydrogels significantly affect cell spreading, proliferation, and differentiation. However, the method of adding hydrophobic units to increase the mechanical strength usually sacrifices the ductility of the hydrogels. Therefore, the development of mechanically enhanced hydrogels with coordinated elongation and toughness is still a topic worthy of attention.
3.1.5. Protein Interaction
In the preparation of hydrogels with natural polymers as the main raw materials, more and more proteins are used, such as gelatin, collagen, silk fibroin, matrix glue, and so on [12,50]. Through non-covalent bond interactions between proteins or polypeptides, conditions such as temperature and phase transition are changed to form protein or polypeptide hydrogels. Spider silk utilizes the sequence differences between eADF3-CTD and eADF4-CTD to self-assemble into β-sheet-rich silk. The precise molecular abundance and composition allow fine-tuning of the solution-gel transition process [51]. With the continuous development of protein engineering, the application range of protein-based hydrogels has become wider and wider, especially the application of recombinant proteins, such as recombinant human collagen (RHC). Deng et al. used RHC conjugated with chitosan to form thermally responsive hydrogels. Experimental results showed that the hydrogels combined with RHC exhibited greater cell infiltration capacity, and could induce more blood vessel formation and accelerate wound healing [52]. Interestingly, both the cell proliferation rate and cell morphologies were found to depend on the hydrogel composition. Increasing the RHC fraction of the hydrogels gradually enhanced the cell spreading and proliferation rate [53].
3.2. Chemical Cross-Linking
At present, most of the hydrogel materials are prepared by chemical cross-linking. Chemically cross-linked hydrogels often have good mechanical properties and stronger stability [54]. They are dominated by the conjugation reaction, free radical polymerization reaction, and the enzymatic reaction.
3.2.1. Conjugation Reaction
The hydrogels cross-linked by the conjugation reaction have become a hot spot. The conjugation reaction can be carried out under relatively mild conditions, including the Michael addition reaction, the Schiff’s base reaction, and the Diels–Alder addition reaction [55]. The Schiff’s base reaction (the condensation of amine and active carbonyl group) is a simple green method in the conjugate reaction [56]. Many polysaccharide molecules contain adjacent hydroxyl groups, such as alginate, starch, hyaluronic acid, and cellulose, which can be oxidized by periodate to form hydrogels through Schiff’s base reactions [29,57,58]. Using oxidized hydroxyethyl starch (O-HES) and modified carboxymethyl chitosan (M-CMCS) as raw materials, an injectable in-situ hydrogel with excellent self-recovery, biocompatibility, biodegradability, and transparency was prepared by the Schiff’s base reaction, which can be injected into irregular-shaped skin defects and formed in situ to shape the contour of different dimensions. The excellent compliance made hydrogels easy to adapt to the wound under different conditions of skin movement, and full-thickness skin defects treated with M-CMCS/O-HES hydrogels demonstrated a higher wound closure percentage, more granulation tissue formation, faster epithelialization, and decreased collagen deposition. It is a promising therapeutic strategy for wound healing [59]. In addition, a novel bioadhesive hydrogel with intrinsic antibacterial properties was prepared by mixing modified hyaluronic acid (HA) and ε -polylysine (EPL) using the Schiff’s base reaction, which can effectively kill bacteria on the surface of wounds, promote angiogenesis, and accelerate wound healing [60]. The QCS/PF hydrogel prepared by the Schiff’s base reaction has antibacterial, antioxidant, hemostatic, adhesion, and mechanical adjustable properties. The gel promotes blood coagulation and the synthesis of ECM components by simultaneously down-regulating TNF-α and up-regulating VEGF, promotes cell signal transduction through electrical stimulation, clears ROS through curcumin, and prevents infection through its antibacterial properties. So as to promote the progress of wound hemostasis, inflammation, and remodeling stages [13].
3.2.2. Free Radical Polymerization
Free radicals can be produced by heating, ultraviolet radiation, high energy radiation, electrolysis, and plasma initiation [61]. The radical polymerization reaction of thermally initiated polymerization and light-initiated polymerization utilizes unsaturated functional groups or photosensitive functional groups to undergo free radical polymerization or cross-linking under the action of heat or light to form covalent bonds [60,62]. Most of the hydrogels prepared by thermally induced cross-linking reactions can be used for a deep wound healing treatment, and the structure is stable and highly controllable [60]. In the photo-initiated polymerization reaction, the precursor containing the photosensitive functional group can be directly polymerized under UV radiation, and the precursor containing the double bond functional group can be polymerized under UV radiation by adding a photo initiator [63]. At present, gelatin, starch, chitosan, sodium alginate, heparin, hyaluronic acid, and other natural polymers are prepared into hydrogel dressings through free radical polymerization, which is widely used in wound dressings. A versatile poly(acrylamide) cellulose nanocrystal/tannic acid–silver nanocomposite (NC) hydrogel integrated with excellent stretchability, repeatable self-adhesion, high strain sensitivity, and antibacterial property, was synthesized via radical polymerization at an ambient temperature. These were merited for the hydrogels to be assembled into a flexible epidermal sensor for long-term human–machine interfacial contact without concerns about the use of external adhesive tapes and bacterial breeding [64]. In situ, PAM/SA/Ag hydrogels were prepared by using silver ions in the presence of ammonium persulfate to catalyze free radical polymerization. Histocompatibility experiment results showed that the hydrogels showed higher expression of CD31 and VEGF, which are related to angiogenesis in wound healing [65].
3.2.3. Enzymatic Reaction
The enzymatic reaction is the cross-linking of natural polymers catalyzed by enzymes such as transglutaminase, tyrosinase, urease, and horseradish peroxidase (HRP) [60,66]. Enzymatic reactions occur under mild conditions, which can prevent the loss of biological activity and rapid gelation, and no harmful substances are produced. At present, the use of enzymatic rapid gelation to prepare antibacterial hydrogels is promising [67,68]. Using HRP and H2O2 to explore the immobilization of low molecular weight hyaluronic acid (LMWHA) derivatives within gelatin-based hydrogels to stimulate the migration of ECs. The result shows that the enzymatic immobilization of LMWHA-Ph within gelatin-based hydrogels represents a promising approach to promote ECs’ motility and further exploitation for vascular tissue engineering applications [69]. Scientists are paying more and more attention to the 3D cell culture of hydrogels, creating a hydrogel network with reversible stiffening/softening capability is important, enzymatic reactions can afford substrate specificity and mild/predictable reaction kinetics [70]. Using transglutaminase to mediate the covalent attachment of HA and PEG macromers, the hyaluronic acid hydrogels formed in situ can specifically regulate the cell phenotype by adjusting their own mechanical and biochemical properties [71].
4. Biomaterials for Preparing Hydrogels
Biomaterials used in tissue engineering or regenerative medicine are generally divided into two categories: natural biomaterials and synthetic materials [72]. Regardless of the source of materials, hydrogel dressings should have low toxicity, good biocompatibility, and facilitate the growth of cells near the wound. In addition, synthetic hydrogel dressings should also have good mechanical properties, biodegradability, moisture retention, antibacterial, antioxidant, non-adhesion, and good air permeability, etc., [73] as shown in Figure 3. Recently, the hydrogel dressings synthesized by natural biomaterials have become the focus of research [74]. Natural biomaterials such as chitosan, collagen, starch, cellulose, sodium alginate, and hyaluronic acid are widely used in the synthesis of hydrogel wound dressings. However, there are some problems such as low mechanical properties, high acquisition cost, small output, and difficult modification. Synthetic materials have specific functions, good mechanical properties, large output, low cost, and rich variety. However, synthetic polymers often lack biological and biodegradable activity and may produce toxic by-products during the reaction, which lead to tissue necrosis. Therefore, researchers are committed to continuously optimizing the performance of synthetic materials and developing hydrogels with different functions.
Figure 3. Schematic illustration of hydrogels’ function for wound repairing.
5. Conclusions and Prospects
In this review, the research progress of hydrogels as a wound dressing is reviewed and summarized. Traditional gauze and cotton dressings often cause secondary damage to the wound when they are removed. Hydrogel dressings are beneficial to the treatment of any type of wound. Researchers have prepared hydrogels with different properties through physical and chemical cross-linking methods, which greatly meet the needs of humans for the treatment of various wound types. In addition, various methods are used to continuously optimize the parameters of the hydrogels to expand the scope of application of the hydrogels. From the point of view of the materials, both natural and synthetic polymers have good biological activity and a wide range of applications. In particular, the mixed-use of various polymers highlights the main role of each component and speeds up wound healing.
In the future, the research of hydrogel dressings will develop towards lower cost and diversified functions. At present, there are various types of hydrogel dressings, and various new hydrogel materials are continuously optimized and their functions are becoming more and more perfect. However, there is still a gap with the ideal hydrogel dressing, especially in the treatment of chronic wounds. In the future, the development of hydrogel dressings can pay more attention to the preparation of materials with strong antibacterial, antioxidant, or slow-release function, or pay more attention to the structural modification of natural polymers. Many factors including materials and methods can affect the properties of hydrogels. The stability, processability, and solubility of the polymer are also important obstacles to optimizing the preparation process. Therefore, researchers can focus on searching for new technologies and materials and consider various influencing factors to improve the function of hydrogel dressings.
References
1. Kim, H.S.; Sun, X.; Lee, J.H.; Kim, H.W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef] [PubMed]
2. Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
3. Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
4. Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, e1902232. [Google Scholar] [CrossRef]
5. da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed][Green Version]
6. Wang, J.; Chen, X.Y.; Zhao, Y.; Yang, Y.; Wang, W.; Wu, C.; Yang, B.; Zhang, Z.; Zhang, L.; Liu, Y.; et al. pH-Switchable Antimicrobial Nanofiber Networks of Hydrogel Eradicate Biofilm and Rescue Stalled Healing in Chronic Wounds. ACS Nano 2019, 13, 11686–11697. [Google Scholar] [CrossRef]
7. Lu, Y.; Wang, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. In-Situ doping of a conductive hydrogel with low protein absorption and bacterial adhesion for electrical stimulation of chronic wounds. Acta Biomater. 2019, 89, 217–226. [Google Scholar] [CrossRef]
8. Qian, Z.; Wang, H.; Bai, Y.; Wang, Y.; Tao, L.; Wei, Y.; Fan, Y.; Guo, X.; Liu, H. Improving Chronic Diabetic Wound Healing through an Injectable and Self-Healing Hydrogel with Platelet-Rich Plasma Release. ACS Appl. Mater. Interfaces 2020, 12, 55659–55674. [Google Scholar] [CrossRef]
9. Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
10. Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration during Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
11. Tavakolian, M.; Munguia-Lopez, J.G.; Valiei, A.; Islam, M.S.; Kinsella, J.M.; Tufenkji, N.; van de Ven, T.G.M. Highly Absorbent Antibacterial and Biofilm-Disrupting Hydrogels from Cellulose for Wound Dressing Applications. ACS Appl. Mater. Interfaces 2020, 12, 39991–40001. [Google Scholar] [CrossRef]
12. Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
13. Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
14. Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research status of self-healing hydrogel for wound management: A review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef]
15. Wang, C.; Wang, M.; Xu, T.; Zhang, X.; Lin, C.; Gao, W.; Xu, H.; Lei, B.; Mao, C. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics 2019, 9, 65–76. [Google Scholar] [CrossRef]
16. Lei, H.; Zhu, C.; Fan, D. Optimization of human-like collagen composite polysaccharide hydrogel dressing preparation using response surface for burn repair. Carbohydr. Polym. 2020, 239, 116249. [Google Scholar] [CrossRef] [PubMed]
17. Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional Tissue-Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
18. Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
19. Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
20. Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
21. Dimatteo, R.; Darling, N.J.; Segura, T. In Situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
22. Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
23. Chen, T.Y.; Wen, T.K.; Dai, N.T.; Hsu, S.H. Cryogel/hydrogel biomaterials and acupuncture combined to promote diabetic skin wound healing through immunomodulation. Biomaterials 2021, 269, 120608. [Google Scholar] [CrossRef] [PubMed]
24. Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
25. Liang, J.; Cui, L.; Li, J.; Guan, S.; Zhang, K.; Li, J. Aloe vera: A Medicinal Plant Used in Skin Wound Healing. Tissue Eng. Part B Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
26. Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef]
27. Li, J.; Guan, S.; Su, J.; Liang, J.; Cui, L.; Zhang, K. The development of hyaluronic acids used for skin tissue regeneration. Curr. Drug Deliv. 2020, 17, 836–846. [Google Scholar] [CrossRef] [PubMed]
28. Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Mutlu, N.; Boccaccini, A.R. Polymeric Hydrogel Systems as Emerging Biomaterial Platforms to Enable Hemostasis and Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000905. [Google Scholar] [CrossRef]
29. Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
30. Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
31. Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef]
32. Ye, L.; Zhang, Y.; Wang, Q.; Zhou, X.; Yang, B.; Ji, F.; Dong, D.; Gao, L.; Cui, Y.; Yao, F. Physical Cross-Linking Starch-Based Zwitterionic Hydrogel Exhibiting Excellent Biocompatibility, Protein Resistance, and Biodegradability. ACS Appl. Mater. Interfaces 2016, 8, 15710–15723. [Google Scholar] [CrossRef]
33. Wang, J.; Wang, L.; Wu, C.; Pei, X.; Cong, Y.; Zhang, R.; Fu, J. Antibacterial Zwitterionic Polyelectrolyte Hydrogel Adhesives with Adhesion Strength Mediated by Electrostatic Mismatch. ACS Appl. Mater. Interfaces 2020, 12, 46816–46826. [Google Scholar] [CrossRef]
34. Yuan, N.; Xu, L.; Xu, B.; Zhao, J.; Rong, J. Chitosan derivative-based self-healable hydrogels with enhanced mechanical properties by high-density dynamic ionic interactions. Carbohydr. Polym. 2018, 193, 259–267. [Google Scholar] [CrossRef] [PubMed]
35. Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef] [PubMed]
36. Li, X.; Peng, X.; Li, R.; Zhang, Y.; Liu, Z.; Huang, Y.; Long, S.; Li, H. Multiple Hydrogen Bonds-Reinforced Hydrogels with High Strength, Shape Memory, and Adsorption Anti-Inflammatory Molecules. Macromol. Rapid Commun. 2020, 41, e2000202. [Google Scholar] [CrossRef] [PubMed]
37. Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef] [PubMed]
38. Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109. [Google Scholar] [CrossRef]
39. Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef]
40. Xiao, J.; Zhou, Y.; Ye, M.; An, Y.; Wang, K.; Wu, Q.; Song, L.; Zhang, J.; He, H.; Zhang, Q.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2001591. [Google Scholar]





